Glioblastoma
Glioblastoma (GBM) is the most aggressive form of brain tumors in adults. A tumor can arise in different locations in the brain and is commonly derived from astrocytes, a particular cell type that makes part of the so-called “Glia” cells. Glial cells are non-neuronal cells that provide support and protection for neurons in the brain. These include oligodendrocytes, astrocytes, ependymal cells and microglia.
GBM tumors make part of the larger group of “gliomas” which are categorized according to their grade, which is determined by pathologic evaluation of the tumor. The most commonly used grading system is provided by the World Health Organization (WHO), under which tumors are graded from I (least advanced disease—best prognosis) to IV (most advanced disease—worst prognosis).
Low-grade gliomas [WHO grade II] are mostly benign and usually have a better prognosis for the patient. However, they have a uniform rate of recurrence and increase in grade over time so should be classified as malignant.
High-grade [WHO grade III–IV] gliomas are undifferentiated or anaplastic and are therefore malignant and carry a worse prognosis. GBM is a grade IV astrocytoma.
Despite intensive treatment including surgery, radiation therapy and cytotoxic chemotherapy using temozolomide (TMZ), the prognosis of GBM patients remains poor, with a median survival of less than 15 months. Virtually all GBM tumors exhibit genetic alterations, including single nucleotide variants, focal or large chromosomal deletions and/or amplifications and fusions, with >50% of tumors containing multiple alterations.
Genetic analyses led to the identification of several GBM tumor subclasses, including the proneural and neural subtypes (which often contains genetic alterations in PDGFRA and IDH1), the classical subtype (often containing EGFR alterations) and the mesenchymal subtype (which contains NF1 alterations). Each of these subtypes exhibits a specific expression profile. Only the classical subtype showed a somewhat better response to the standard-of-care compared to the others.
Through consortia such as The Cancer Genome Atlas (TCGA) the major genetic pathways that get altered in GBM were identified and include (i) RTK receptors (EGFR, PDGFR, MET and FGFR) and their downstream signaling regulators (PIK3CA/PTEN/NF1), (ii) the p53 pathway (p53, MDM2/4, CDKN2A), (iii) various members of the cell cycle control (CDKs, Cyclins, RB1) and (iv) Chromatin remodeling (KMT2C, IDH1). Major efforts have been invested in targeting these pathways, but in spite of numerous therapeutic trials, treatment options remain limited and inefficient.
The complex genetics of GBM may explain why targeting a single signaling pathway may not be sufficient to stop tumor growth and may require combined treatments. Second, due to the large variety in combinations of genetic alterations in GBM, only small groups of patients could be responsive to particular treatments, thereby making clinical trials more prone to fail, even though some patients did respond well. Also, the blood brain barrier (BBB) may prevent therapeutics to actually reach the brain.
At the LPCM, we are trying to contribute to a better understanding of the GBM tumors and how to treat them.
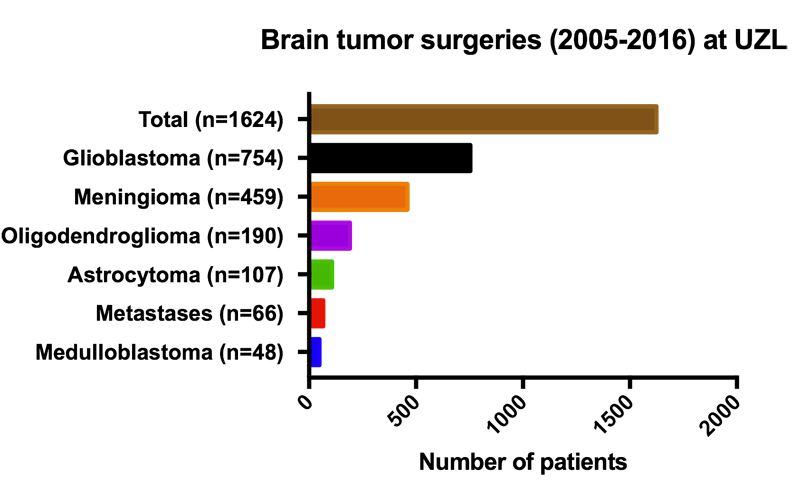
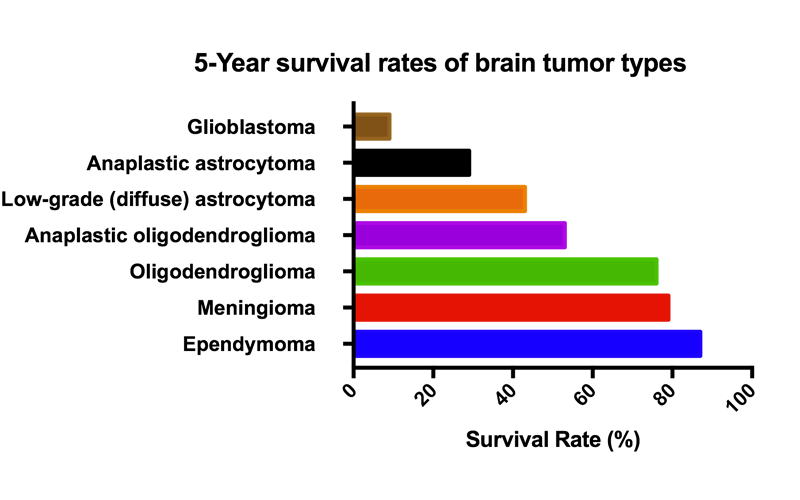
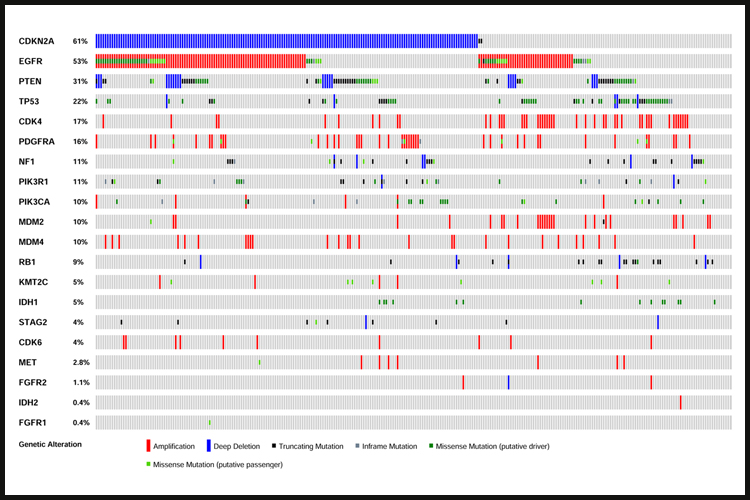
![IMG_0535[1].JPG](https://images.squarespace-cdn.com/content/v1/58b2f2f403596e617b31a171/1529063265492-5XP14HTQ7NKOL4M9F848/IMG_0535%5B1%5D.JPG)
![IMG_0521[1].JPG](https://images.squarespace-cdn.com/content/v1/58b2f2f403596e617b31a171/1529063234484-MJW93L6ZMADG45PASS25/IMG_0521%5B1%5D.JPG)



